Orthofix recognizes the importance of collecting data from centers around the globe as we continue to monitor the clinical performance and safety of the M6-C artificial cervical disc. Please see some of our key data points below.
Patient Focused.
Proven Results.
Real World Evidence
70,000+ Devices Implanted
The M6-C artificial cervical disc is designed to maintain the natural behavior of a functional spinal unit by replicating the biomechanical characteristics of the native disc. The M6-C disc has established a robust clinical history with over 70,000 devices implanted in over 20 countries.

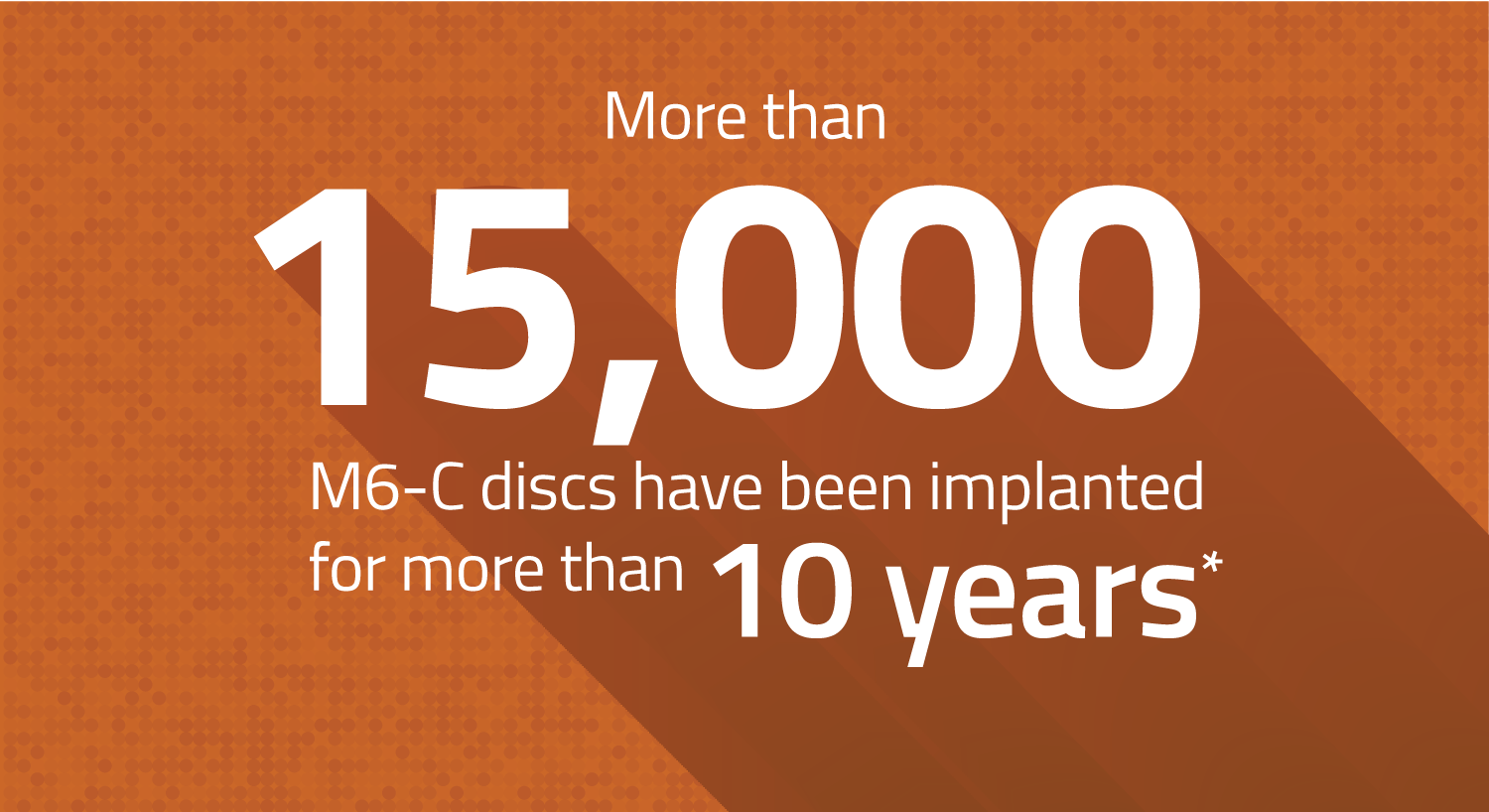
Over 15,000 Devices Have Been Implanted For More Than 10 Years
First introduced outside the U.S. in 2006, the M6-C disc has a significant clinical history. Of the 70,000 devices implanted globally:
- 40,000 discs have been implanted for more than 5 years
- 30,000 discs have been implanted for more than 7 years
- 15,000 discs have been implanted for more than 10 years
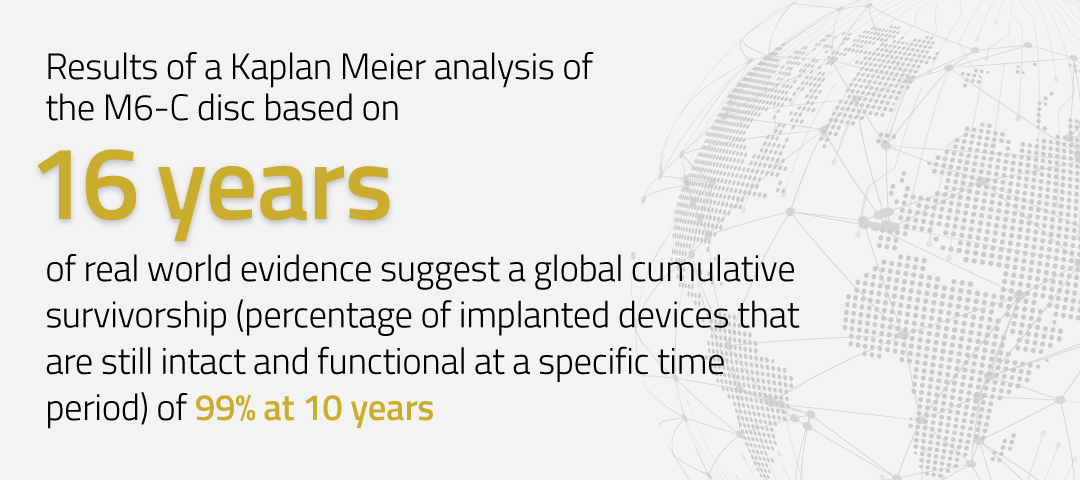
99% Device Survivorship at 10 Years
Results of a Kaplan Meier analysis of the M6-C™ artificial cervical disc based on 16 years of real world evidence suggest a global cumulative survivorship (percentage of implanted devices that are still intact and functional at a specific time period) of 99% at 10 years, consistent with other, proven, joint-arthroplasty devices such as hip and knee replacements.

Independent Analysis
Orthofix has engaged an independent lab to thoroughly investigate all returned devices to better understand the science and long-term clinical performance of the M6-C disc. To the best of our knowledge, we are the only company that performs this type of in-depth analysis.
In FDA-mandated pre-clinical wear testing, the M6-C™ artificial cervical disc demonstrated very low rates of wear; in fact, reported rates were a fraction of the rates reported for other FDA-approved devices.
A prospective, non-randomized, concurrently controlled clinical trial, the M6-C IDE study was conducted at 23 sites in the U.S. The study evaluated the safety and effectiveness of the M6-C artificial cervical disc compared to ACDF for the treatment of single-level symptomatic cervical radiculopathy with or without cord compression. The M6-C disc received U.S. FDA approval in February 2019 based on the results of this study.
About the M6-C Artificial Cervical Disc
The M6-C artificial cervical disc is a next-generation intervertebral disc designed to restore physiologic motion to the spine and is indicated as an alternative to cervical fusion. The device is comprised of ultra-high molecular weight polyethylene fiber wrapped in a specific pattern, with multiple redundant layers that create a fiber matrix (artificial annulus). The fiber is then wound around a polycarbonate urethane polymer core creating an artificial nucleus. Like a natural disc, this unique construct allows for shock absorption at the implanted level and provides a controlled range of motion when the spine transitions in its combined complex movements. The M6-C artificial cervical disc is the only disc available in the U.S. with these features.
Download Infographic